On September 3, the novel food ingredient sodium hyaluronate was publicly solicited for comments, and the deadline of comments is October 3, 2020.
In 2008, Sodium Hyaluronate has been approved as a novel food ingredient for be used only in health food.
At present, sodium hyaluronate and products with sodium hyaluronate as the main ingredient are allowed to be added to food or dietary supplements in Japan, Korea, the United States, the European Union, Australia, New Zealand and Brazil.
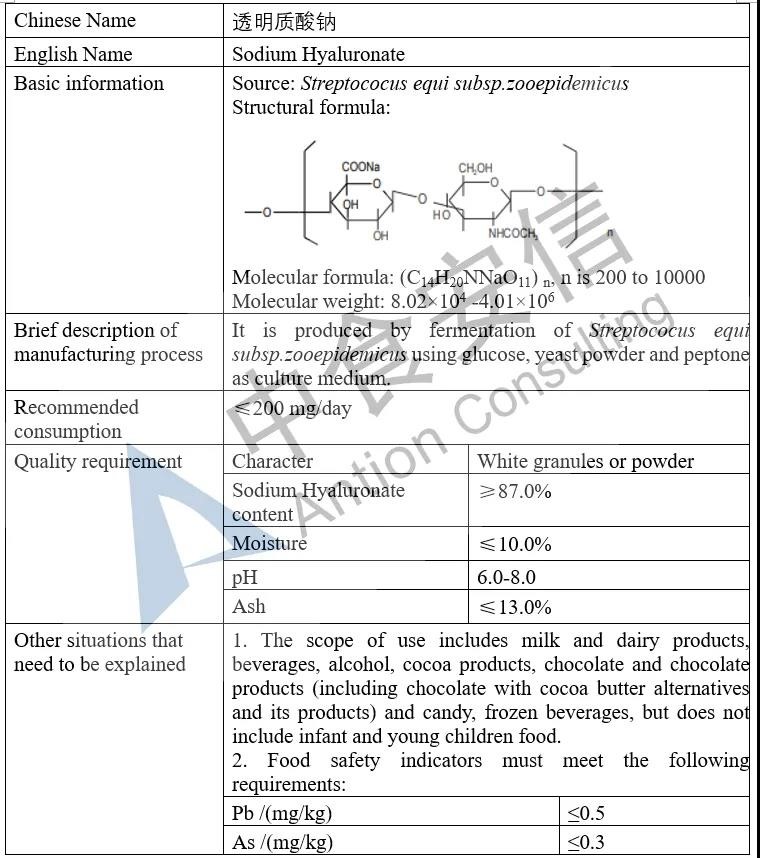
Based on the approval of use in other countries and international organizations, it is planned to expand the use of Sodium Hyaluronate to include milk and dairy products, beverages, alcohol, cocoa products, chocolate and chocolate products (including chocolate with cocoa butter alternatives and its products) and candy, frozen beverages, but does not include infant and young children food.
Proposed announcement on novel food ingredient Sodium Hyaluronate