The development of food science and technology and industry and the globalization of trade have promoted the diversity of food sources in the Chinese market. One of the more obvious trends is the increasing globalization of the supply of food and ingredients. The types and sources of food ingredients are also expanding. Some food ingredients that do not have traditional dietary habits in China are gradually coming to consumers’ attention. In order to regulate the development and application of food ingredients and ensure food safety, these ingredients must be declared and evaluated as novel food ingredients and approved by the National Health Commission (hereinafter referred to as NHC) before they can be marketed for sale. To promote enterprises' understanding of novel food ingredients and avoid food compliance issues, this passage introduces the novel food ingredients management system in China.
Introduction of Novel Food Ingredients
The term "novel food ingredients" was formerly known as new resource foods, and it originally came from Article 22 of the Food Health Law (Trial) issued by China in 1983 ("Food produced from new resource foods must be approved by the Department of Food Health" ), thereby the basic management system for new resource foods was preliminarily determined. Since then, the expression "novel food ingredients" was used in Article 44 of the Food Safety Law issued and implemented in 2009. In 2013, with the issuance and implementation of the Administrative Measures on the Safety Review of Novel Food Ingredients, "New Resource Food" was officially renamed as "Novel Food Ingredients", which was continued to use until now. At present, the current Administrative Measures on the Safety Review of Novel Food Ingredients has been implemented for nearly 13 years. It is predicted that the relevant laws and regulations on novel food ingredients will be revised in the future, and the management system and regulations will be further clarified.
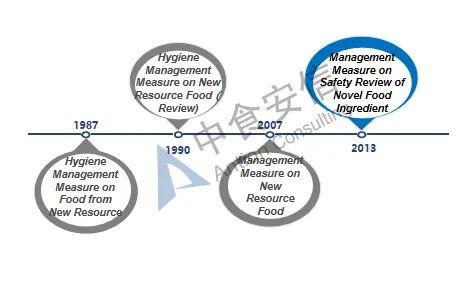
Figure 1: Development of novel food ingredients regulations
From 1987 to 2007, both final food products and food ingredients can be classified according to the new resource foods. With the update of management regulations, since December 2007, the management objects of such regulations have been limited to food ingredients. In addition, the definition and scope of such ingredients have also gradually been clarified and refined. At the same time, with the improvement of relevant management regulations, the currently effective Administrative Measures on the Safety Review of Novel Food Ingredients also clarifies four types of non-novel food ingredients that do not meet the declaration requirements: 1. Substances that do not have the characteristics of food ingredients; 2. Substances that have been listed in GB 2760 and GB 14880 (i.e. food additives and nutritional fortifiers); 3. Substances for which NHC has made a decision not to grant administrative permission; 4. Other substances that do not meet the requirements of relevant laws and regulations.
From the perspective of approval, from 2008 to 2019, the total number of approved novel food ingredients is 130 (Figure 2 for details and Table 1 for the list of approved ingredients).
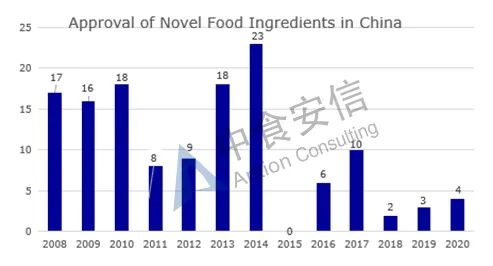
Figure 2: Approval of novel food ingredients in China over the years
Table 1: List of novel food ingredients approved by China over the years
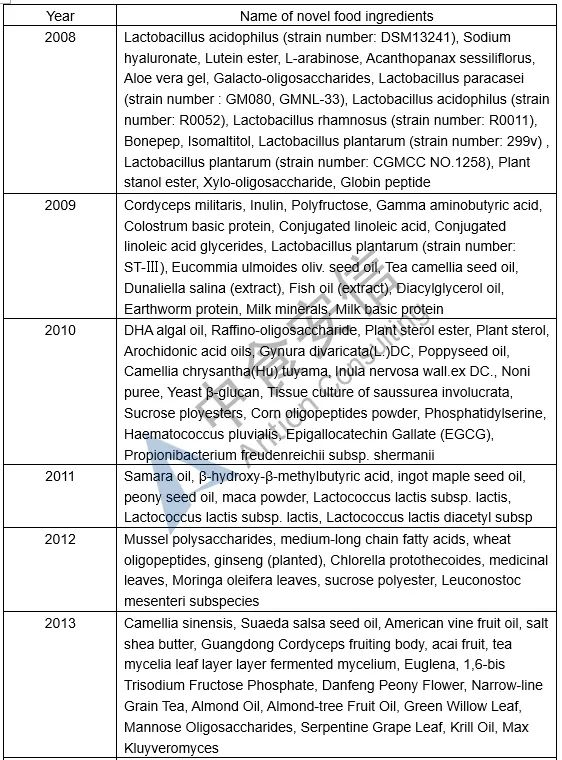
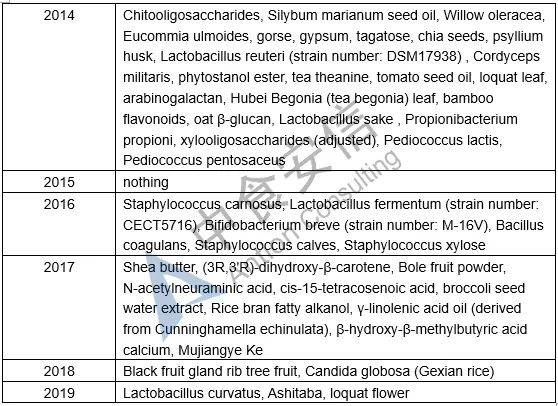
Next week, we will introduce the requirements for novel food ingredients application materials. Please pay attention to us!
Contact Information
Lillian Fan
+86-10-51301566
Lillian.fan@ruibole.com
Relevant Reading
Approval Process of Novel Food Ingredients
Requirements for Novel Food Ingredients Application Materials
Introduction of Food Related Application & Registration Services