Main changes of the revised national standards
1) Separate standards for Stage 2 and Stage 3 formula
The Older Infant and Young Children Formula (GB 10767-2010) is divided into two standards: the Older Infant Formula (GB 10766-2021) and the Young Children Formula (GB 10767-2021), which is in consistency with the trend of Codex standards.Note: Stage 1 refers to infants aged 0 to 6 months, Stage 2 to older infants aged 6 to 12 months, and Stage 3 to young children aged 12 to 36 months.
2) Adjustment of macronutrient indicators
The macronutrients (protein, carbohydrate and fat) are revised in line with nutrient needs of infants and young children in China. Firstly, the requirements for protein content of older infant and young children formula were adjusted, and whey protein content of older infant formula were increased. Secondly, the requirement for carbohydrate content in older infant formula was adjusted to be consistent with that of infant formula; Thirdly, the requirement for lactose content in older infant and young children formula were increased, and the use of sucrose in infant and older infant formula was explicitly restricted.
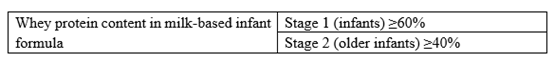
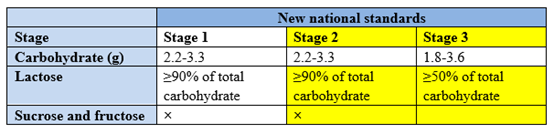
3) Adjustment of upper and lower limits of vitamins and minerals
Firstly, the minimum values of some indicators were set to ensure the adequacy of nutrient intake. Secondly, the maximum values of some indicators were set to ensure the safety of nutrient intake; Thirdly, considering the absorption and utilization of iron, zinc and phosphorus of soy-based infant formula, separate requirements for the content of iron, zinc and phosphorus in soy-based products were added.

* The VD content in Stage 1 is significantly increased
4) Optional ingredients were changed to essential ingredients
Choline, selenium and manganese play an important role in the growth and development of infants and young children. Based on the actual situation of addition of the above nutrients in products on the Chinese market, choline in infant and older infant formula was adjusted from optional ingredient to essential ingredient, and manganese and selenium in older infant formula were adjusted from optional ingredients to essential ingredients.


5) Other special requirements
The species (strain number) should conform to the list of species permitted to be used in infant and young children food issued by the former Ministry of Health, the former National Health and Family Planning Commission and the National Health Commission.
It is allowed to add galactooligosaccharide and other substances that are both nutritional fortification substance and novel food in infant formula. If for nutritional fortification, its use should meet the requirements of the Standard for the Use of Nutritional Fortification Substances in Foods (GB 14880); If used as food materials, it should conform to the provisions of related announcements on novel food.
Related requirements for imported infant formula
1) Since the implementation of the new national standards, overseas enterprises should produce food exported to China in accordance with the new national standards. Products produced or imported before the implementation of the new national standards and in line with the provisions of the original national standards may, according to the provisions of the national standards and the rules of the World Trade Organization, continue to be imported and sold within the shelf life. If there are any special provisions, the special provisions should prevail.
2) For better coordination between the old and new standards, the consignee or its customs declaration agent of imported goods in China must fill in the "date of production" when applying to the Customs the above products to enter into China. The date of production should be consistent with that on the label of the product, which should be expressed with 8 digits in the order of year (XXXX), month (XX) and date (XX).
The above requirements should be implemented as of the implementation date of each new national standard.
Labeling
True and accurate
1) The labels of infant formula milk powder products should comply with food safety laws, regulations, standards and relevant provisions on product formula registration. The contents of the labels should be true, accurate and legible, and should not contain false, exaggerated or misleading words, graphics or absolute contents.
Cautious in claims
2) Content claims and functional claims should not be made for infant formula milk powder for 0 ~ 6 months of age. Content claims and functional claims should not be made on the essential ingredients of older infant and young children formula milk powder for above 6 months of age, while content claims and functional claims permitted by national food safety standards may be made on the optional ingredients on non-main display panel in text form.
Main display panel
3) The main display panel of the product label should be marked with the product name, net content (specification) and registration number. Graphics which meet the requirements of the national food safety standards and are not misleading to consumers may also be marked. Registered trademarks may also be marked at the corner of the main display panel. No other content should be marked.
Animal origin
4) Where the name of a product contains the wording of animal origin, the raw milk, milk powder, whey powder and other milk protein sources should all come from that specie. If the same milk protein material used comes from two or more animals, the proportion of material from each animal origin should be indicated in the ingredients list.
Compound ingredients
5) The compound ingredients in the ingredients list of the product label should be marked in strict accordance with the requirements of the national food safety standards. If an ingredient is a compound ingredient consisting of two or more other ingredients (excluding compound food additives), the name of the compound ingredient should be indicated in the ingredients list, followed by the original ingredients of the compound ingredient in parenthesis in descending order of the amount added.
Precise expression
6) The recommended amount of intake or feeding on the product label should be based on scientific evidence and precise in expression, and such wording as "must" and "strict" should not be used.
Source: Qingdao Customs 12360 hotline
Note: This article is compiled by Antion, please indicate our source if reprint it.