On January 13, 2022, the State Administration for Market Regulation issued a series of soliciting opinions on health food regulations, which are the Catalogue of Health Functions Allowed for Health Food Claims Non-Nutrient Supplements (2022 Version) (hereinafter referred to as the Catalogue) (Draft for Comments) and four supporting documents. The four supporting documents include Guidelines for Inspection and Evaluation of Health Food Functions (2022 Version) (Draft for Comments), Inspection and Evaluation Method of Health Food Functions (2022 Version) (hereinafter referred to as Inspection and Evaluation Method) (Draft for Comments), and Guidelines for Ethical Review of People Trial of Health Food (2022 Version) (Draft for Comments) and Interpretation of Health Food Function Claims (2022 Version) (Draft for Comments). The deadline for comments is February 12, 2022. In order to help enterprises better understand the relevant requirements, Antion analyzed and interpret the main changes and impacts of the Draft.
1. Adjust the Catalogue of Health Functions Allowed for Health Food Claims
SAMR once issued an announcement on soliciting opinions on adjusting the health functions of health foods on March 28, 2019 for the first time. Then, SAMR solicited opinions on November 11, 2020 secondly. SAMR made adjustment based on the feedback, and this is the third time to solicit opinions. The purpose is to further strengthen the management of health food function claims and avoid false propaganda.
Majority adjustments include:
1.1 Eliminate three categories of health functions
The Draft adjusts the original 27 categories of health functions into 24 categories, and eliminates the original three categories of health functions, which includes "facilitating milk secretion", "improving growth and development", and "improving skin oil content". Meanwhile, since the former Ministry of Health no longer accepts the approval of such health functions as suppressing tumor, assisted suppressing tumor, anti-mutation and anti-aging, these health function claims are not included in the Catalogue.
1.2 Standardize the expression of health functions
The Draft regulates the expression of the remaining 24 categories of health functions. For example, "assisting blood sugar reduction" is standardized as "function of helping to maintain a healthy blood sugar level", and "assisting blood pressure reduction" is standardized as "function of helping to maintain a healthy blood pressure level", etc. And the expression "function of helping to…" is added to most of the original health function claims. It not only solves the problem of false or exaggerated propaganda of health food in the market due to inaccurate expressions of health functions, but also guide consumers to correctly understand the function of health food to a great extent.
Figure 1 The Corresponding Adjustment Specification Table of Health Function Claims
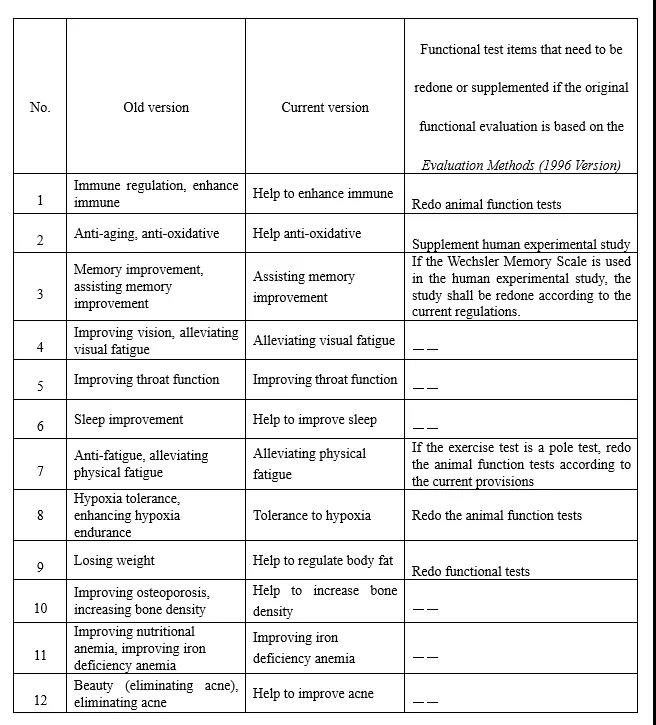
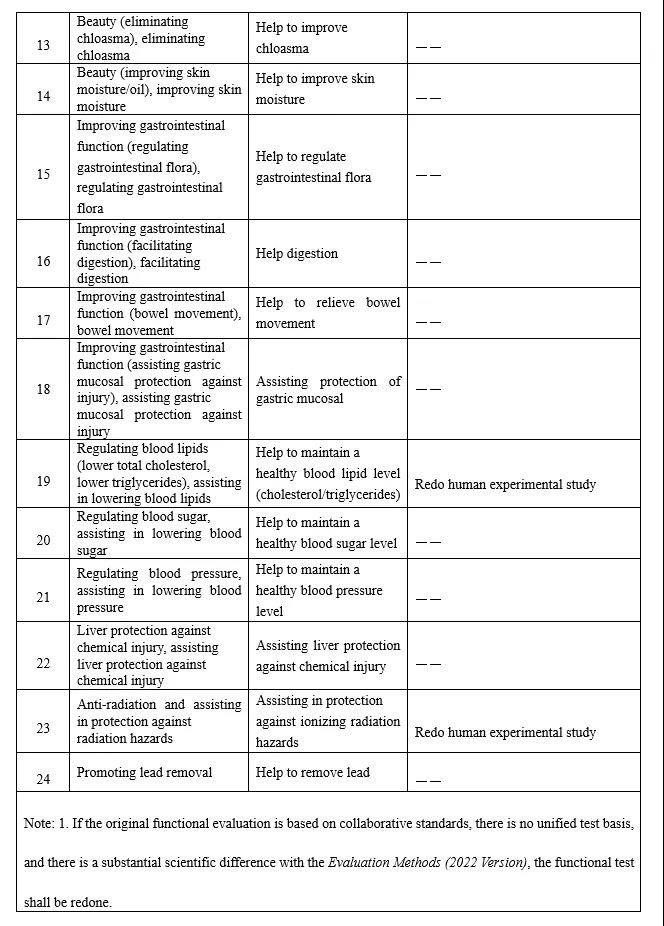
2. Increase the Interpretation of Health Function Claims
The Interpretation of Health Food Function Claims (2022 Version) (Draft for Comments) increases the interpretation of claims for 24 categories of health functions. Previously, opinions were also solicited on the interpretation of health function claims for nutrient supplements health food. This can be said to be in line with the interpretation of health function claims for nutrient supplements.
3. Fill the Gap of Missing Evaluation on Some Health Food Functions
In addition to the adjustment of health functions, the Draft also releases the Evaluation Method of Health Food Functions (2022 Version) (Draft for Comments) and Guidelines for Evaluation of Health Food Functions (2020 Edition) (Draft for Comments), which fill the gap that there has been no evaluation method for some health food functions.
4. Management of Connection between New and Old Health Function Claims and Evaluations
The Function Catalogue is implemented from the date of announcement, and the functions in the Catalogue of Health Food Raw Materials published are automatically adjusted accordingly. Among them, for products that do not need to be redone or supplemented with functional test items, and are already in the process of accepting review and approval, the technical review agency will directly adjust the health function claims and related content of the instructions, and the applicant does not need to make corrections. Health food manufacturers shall revise related functional descriptions according to the Function Catalogue when reprinting their packaging, labels, and instructions, and there is no need to apply for a separate registration change, and the listed products can be sold until the end of the shelf life. According to the substantial scientific similarities and differences of inspection and evaluation methods in different periods, if functional tests need to be redone or supplemented, the registration change shall be applied, and a new registration certificate shall be issued if the requirements are met.
For the products in production and sale that need to be redone or supplemented with functional test items, the post-marketing population functional evaluation research report issued by the third-party inspection agency can also be provided. Data collection, management, and analysis shall be standardized, true, and traceability and provide effective technical support for health functions of product.
5. Disposal of products with health functions not listed in the Function Catalogue
In addition to inhibiting tumor, helping to inhibit tumor, anti-mutation, delaying aging, promoting lactation function, improving growth and development function, improving skin oil, the approved health functions that have not been cancelled or included in the catalogue of health functions shall be transformed according to the connection between the new and old health functions claims and the latest evaluation requirements; If it cannot be transformed, according to the Administrative Measures for Catalogue of Health Food Raw Materials and Health Function Catalogue, a suggestion to be included or adjusted to the catalogue of health function shall be put forward to the review agency, and those that meet the requirements shall be included or adjusted in the Function Catalogue.
6. Inspection Basis of Inspection Agency of Health Food Registration
The inspection agency of health food registration shall carry out various work such as product function evaluation tests according to the Inspection and Evaluation Method for the related product inspection applications for registered health food accepted from the date of announcement. The inspection report shall be issued and submitted in accordance with the registration requirements.
Conclusion
The issuance of the Draft has a large impact on registered health food. It not only fills in the lack of regulations, but also gradually improves the legal system, which is conducive to the rapid development of the health food market. In addition, combined with the common problems and shortcomings in the current health food market, the Draft also makes relevant deletions and adjustments to health functions, which is more convenient for consumers to understand and also helpful for enterprises’ promotion, a win-win situation for consumers and enterprises, and more conducive to the healthy development of the whole industry. Through the interpretation of the Draft, Antion expects to help enterprises and industry personnel better grasp the changing trends and provide guidance for the next step in the work. Besides, Antion can provide consulting services such as consultation of standard and regulation, and food labeling review, etc. If you have any questions, please feel free to contact us!
Hongtao Fei
Tel: 010-51301566
email: feiht@ruibole.com
Source: Antion
Note: This article is compiled by Antion, please indicate our source if reprint it.