The biggest amendment to this standard is the introduction of "mass food fortification" and "voluntary food fortification". Along with the introduction of the two concepts, the amendment stipulates and revises the types and usage amounts of nutritional fortification substances that are permitted to be used in the two cases.
As per the notice released by China National Center for Food Safety Risk Assessment (CFSA) on July 31, China is now soliciting public feedbacks on the 2nd draft of GB 14880-2012 National Food Safety Standard Standard for the Use of Nutritional Fortification Substances in Foods. The deadline for comments is August 10, 2023.
Major Changes Compared to the 1st Draft
1. Revisions to the main body
l The expression raised in the 1st draft "The foods in Chinese Dietary Guidelines whose consumption volume are suggested to be reduced should not be taken as the carrier of nutrition fortification." is changed back into "The choice of food carrier should be in line with the requirements of rational diet advocated by dietary guidelines and/or relevant nutrition policies".
l There is a new requirement that the allowable amino acids and compound sources used in foods for special dietary uses shall comply with the provisions of Appendix E and (or) relevant product standards.
l Due to the addition of Appendix E—Allowable Amino Acids and Compound Sources Used in Foods for Special Dietary Uses, the appendix for the explanation of food categories (names) is updated to Appendix F.
2. Revisions to Appendix A: Rules for Using Nutritional Fortification Substances in Mass Food Fortification
l Table A.1 specifies the types and amounts of nutrients that should be used in priority in the case of mass food fortification and involved food categories. The 2nd draft revises the food category that can be fortified, and only make changes to dairy category as below. Fermented milk and flavored fermented milk is deleted from this Table.
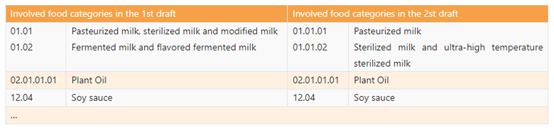
l Table A.2 specifies the types and amounts of nutrients that can be used optionally for mass food fortification and involved food categories. Just like the revision in Table A.1, the category "fermented milk and flavored fermented milk" is also deleted from Table A.2. Moreover, Table A.2 introduces four nutrients: L-Lysine, γ-linolenic acid, casein calcium peptide, and casein phosphopeptides.
3. Revisions to Appendix B: Rules for Using Nutritional Fortification Substances in Voluntary Food Fortification
l It is emphasizes that when both raw materials and finished products allow the use of a certain fortifier, the variety and amount of nutritional fortification substance should meet the fortification requirements of the finished product.
l Table B.1 specifies the types and usage amounts of vitamins and minerals that are permitted for voluntary food fortification. The 2nd draft newly adds modified milk, flavored fermented milk, novel soy products (such as vegan soy-based meat) and sugar-free candy into involved food categories. Use amount of some nutrients are revised a bit. In addition, two items, choline and inositol, are moved from Table B.2 to Table B.1.
l Table B.2 specifies the types and usage amounts of other components that are permitted for voluntary food fortification. Yeast β-glucan and γ-linolenic acid are added into this table. Casein calcium peptide and casein phosphopeptides become two separate items (same as the current GB 14880-2012 version). Use amount of some components are revised.
4. Revisions to Appendix C: Allowable Nutritional Fortification Substances and Their Compound Sources
l Bone meal (fine fresh bone meal) and γ-linolenic acid are kept in Appendix C in the 2nd draft.
l Casein calcium peptide and casein phosphopeptides become two separate items.
l There are also some revisions to the names of substances and the notes for compound sources.
5. Revisions to Appendix D: Allowable Nutritional Fortification Substances and Compound Sources Used in Foods for Special Dietary Uses.
l The 2nd draft adds seaweed iodine, sodium fluoride and potassium fluoride to Table D.1.
l Due to the adjustment of food categories in Appendix F, the category number of foods for special medical purpose targeted at infants (infant FSMP) is revised into 13.01.04 and becomes a subcategory under 13.01. Therefore, the category type in Table D.2 is revised accordingly. In another word, there’re no substantial changes with Table D.2.
6. Revisions to Appendix E and F are mentioned as above.
Major Revisions Compared to the Current 2012 Version
1. Terms and expressions are optimized.
l The definition of "foods for special dietary uses" is deleted for it has already been stipulated in another standard GB 13432 National Food Safety Standard Labeling of Prepackaged Foods for Special Dietary Uses.
l As many nutrition policies raised requirements for "the reduction of sugar, fat and salt", etc., the expression foods in Chinese Dietary Guidelines whose consumption volume are suggested to be reduced should not be taken as the carrier of nutrition fortification. was changed into "The choice of food carrier should be in line with the requirements of rational diet advocated by dietary guidelines and/or relevant nutrition policies".
2. Names and numbers of food categories are adjusted.
The amendment to food categories involves dairy and dairy products, fruits, vegetables, beans, edible fungus, algae, nuts and seeds, cereal and cereal products, bakery products, meat and meat products, beverage, etc.
Taking 01.0 dairy and dairy products (excluding 13.0 foods for special dietary uses) as an example, the draft newly adds specific use conditions of nutritional fortification substances into three new subcategories, namely, "01.04 condensed milk and its analogue", "01.05 cream and its analogue" and "01.07 instant flavored dessert or its prepared products with milk as the main ingredient (excluding ice cream and flavored yogurt)".
3. Requirements for "mass food fortification" and "voluntary food fortification" are regulated.
The biggest amendment to this standard is the introduction of "mass food fortification" and "voluntary food fortification". The two terms are referenced from CAC/GL 09-1987 General Principles for the Addition of Essential Nutrients to Foods and Guidelines on Food Fortification with Micronutrients by WTO.
a. Mass food fortification is the addition of one or more micronutrients to specific foods that are widely consumed by the public. It is usually organized and implemented by government departments. Along with the introduction of this concept, Appendix A of the amendment stipulates the types and usage amounts of nutritional fortification substances that are permitted to be used for mass food fortification. Involved categories include some dairy products, vegetable oil, rice, wheat flour and soy sauce.
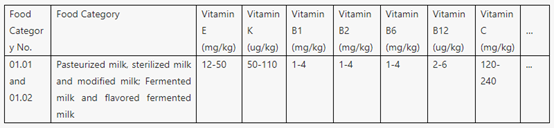
l To be specific, Table A.1 specifies the types and amounts of nutrients that should be used in priority in the case of mass food fortification.
Part of Table A.1

l Table A.2 specifies the types and amounts of nutrients that can be used optionally for mass food fortification. Nutritional fortification substances listed in Table A.2 can only be used after foods are fortified with substances in Table A.1 first.
Part of Table A.2
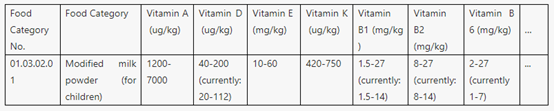
b. Voluntary food fortification is the voluntary addition of one or more micronutrients and/or other nutrients to foods other than those subject to mass food fortification. It is usually at the discretion of the manufacturer. Appendix B specifies and revises the types and usage amounts of nutritional fortification substances that are permitted for this purpose. For example, the use amount of DHA is changed from percentage into a specific use amount. Moreover, the amendment newly adds a requirement to regulate the situation when food is produced with auxiliary materials that have been fortified with nutritional fortification substances.
Part of Table B.1
4. Requirements for permitted nutritional fortification substances and usage amount is revised.
Many requirements for permitted nutritional fortification substances are revised and the requirements for substances approved in official notices in the past years are added in the Appendix.
Source: CFSA and Chemlinked
Note: This article is compiled by Antion. Please indicate the source for reprint.