On March 9, National Medical Products Administration (NMPA) has released the information on the Formula Registration Result of Infant and Young Children Formulas Milk Powder (short for IF). 9 formulas applied by the French factories LAITERIE DE MONTAIGU SAS SABOURIN have been approved, which is the first batch of imported IF that has been registered since April 2019. The other 3 formulas are applied by Junlebao Junheng Dairy Co., Ltd.
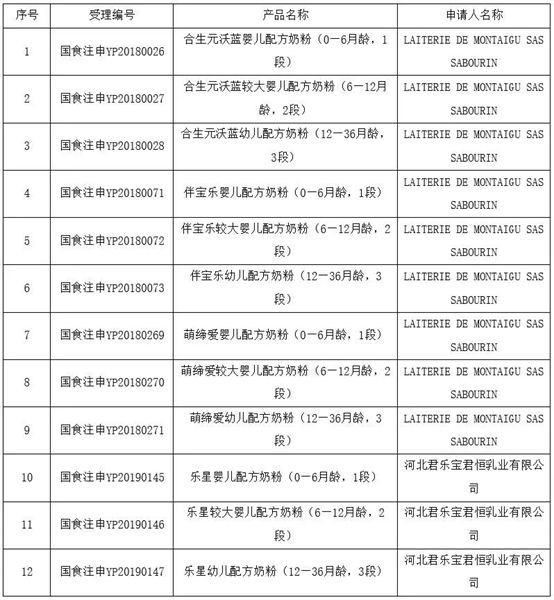
Among them, the whole registration process of Babybio Infant and Young Children Formulas Milk Powder (Stage 1, 2, and 3) and Modilac Infant and Young Children Formulas Milk Powder (Stage 1, 2, and 3) is supported by Antion.
As a professional institution engaged in consulting services for food industry, Antion (Beijing) Information Consulting Co., Ltd. has abundant resources superiority and the specialized technical ability, and also has provided infant formula registration agent service for many customers, which include:
l Dynamic monitoring and interpretation analysis of infant formula registration related laws and regulations
l Feasibility analysis of infant formula registration
l Preparation and review of application materials
l Whole-process communication guidance (good English communication skills)
l Factory on-site inspection support
l Other related work (such as experimental arrangement)
If you have interest, please contact us!
Lillian Fan
86-10-51301566
Lillian.fan@antionchina.com