Last week, we introduced the requirements for novel food ingredients application materials. Now, let's take a look of the application process of novel food ingredients.
Approval process of Novel Food Ingredients
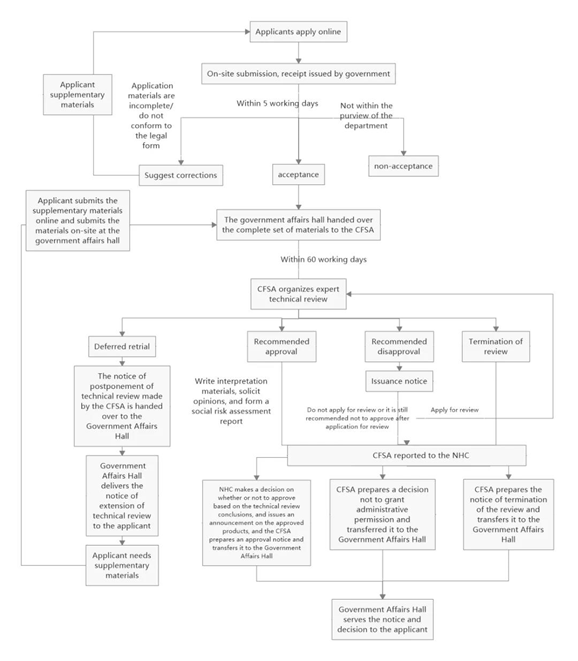
The specific approval process for novel food ingredients can be seen in Figure 1
Source: NHC
Figure 1: The flow chart of novel food ingredient approval
Review Conclusion
As seen from Figure 1, the review conclusion for novel food ingredients are divided into four situations, which are deferred retrial, recommended approval, recommended disapproval and termination of review. The specific explanations for these situations are as follows.
Deferred retrial
If the submitted application materials need to be modified or supplemented, or if further verification tests or scientific demonstrations are required, the NHC shall issue the "Notice of the Administrative Licensing Technical Review Extension". The applicant shall supplement and submit the missing application materials in accordance with the notice, then carry on another expert review.
Recommended approval
If the applied substance passes the review of the expert organization and the review conclusion is "Recommended Approval", the NHC will solicit opinions from the society for 30 days. After that, the NHC will make an analysis on the opinions, and finally issue the "Notice of Administrative Licensing Technical Review Conclusions".
Recommended disapproval
If the applied substance does not meet the definition of novel food ingredients (e.g., it does not have the characteristics of novel food ingredients), or does not meet the required nutritional requirements, or the safety cannot be guaranteed, the technical review conclusion will be "Recommended Disapproval". The NHC will issue the "Notice of Opinions on Administrative Licensing Technical Review" to the applicant. If the applicant disagrees with this conclusion, the applicant can ask for re-check within 30 days. If the conclusion for re-check is still "recommended disapproval", or the application for re-check is not submitted within the limited time, the NHC will issue the "Decision on Rejection of Administrative Licensing" to the applicant and inform the reasons for disapproval.
It should be noted that “Recommended Disapproval” can also occur if the applied material or sample is not authentic. In addition, according to the above definition of non-novel food ingredients, substances that do not have administrative permission cannot be applied as novel food ingredients again.
Termination of review
If the applied substance is common food ingredient, or is substantially equivalent to the common food ingredient or the approved novel food ingredient, the NHC will issue the "Notice of Administrative Licensing Termination of Review" to the applicant and inform the reason.
The word “substantial equivalence” comes from the New Resources Food Management Measures in 2007, which refers to, if a newly applied food ingredient is the same with a food or a new published novel food ingredient in terms of species, source, biological characteristics, main constituents, edible parts, amount of use, range of use and application group, etc., and the process and quality requirements are basically the same, they can be considered as equally safe and substantial equivalent.
Contact Information
Lillian Fan
+86-10-51301566
Lillian.fan@ruibole.com
Relevant Reading
Basic information for novel food ingredients
Requirements for Novel Food Ingredients Application Materials
Introduction of Food Related Application & Registration Services